Getting started on your project can be confusing. Following is information that will
guide you along the pre-IRB/pre-OSP submission process. Links are embedded in
graphs and text to provide you with supporting documentation to assist in making
decisions.
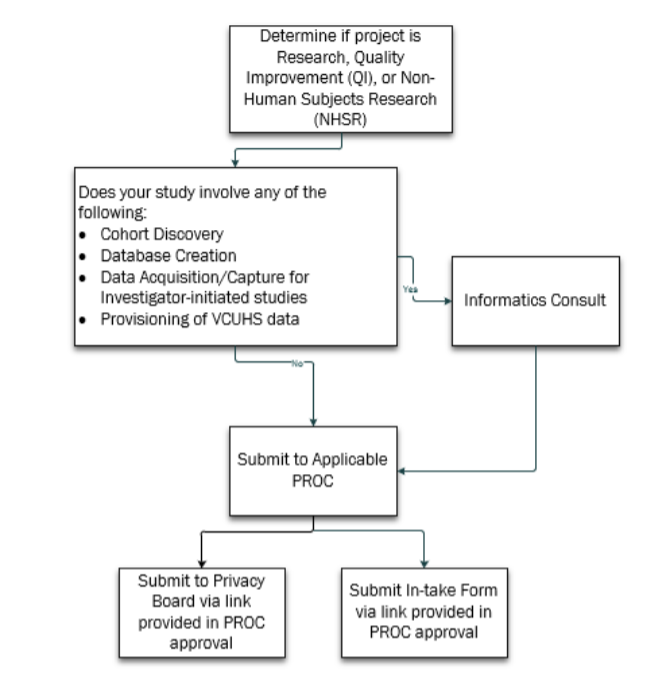
The first step is to determine if your project is Research or Quality Improvement (QI.) Once that decision has been made, if your study requires cohort discovery, database creation, any type of data acquisition or capture for an investigator-initiated study or provisioning of VCUHS data, then you will need to have an Informatics Consult. Once the Informatics Consult has occurred and your data plan has taken shape, you will then submit to the applicable PROC. If your study does not require any type of cohort discovery, database creation, any type of data acquisition or capture for an investigator-initiated study or provisioning of VCUHS data, then you can proceed directly to PROC submission.
Determine if your project is Research
Making a determination if a project is Research or QI can be difficult. When you add
NHSR into the decision, it becomes even more of a challenge. First, let’s review the
definitions. What is Research, what is QI, and what is Non-Human Subjects Research
(NHSR)?
Research is a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge (45 CFR 46.102) Systematic: Having or involving a system, method, or plan Investigation: Searching inquiry for facts; detailed or careful examination Develop: to form the basis for a future contribution Contribute: giving/supplying results Knowledge: truths, facts, and/or information Generalizable: Universally or widely acceptable Generalizable Knowledge: Universally or widely acceptable truths, facts, and/or information
A better way to think about it is research is when you use a systematic approach to discover something that is unknown. Intended for widespread applicability, time consuming, may not directly affect patient care within a specific setting.
To help you determine if your study is Research or QI, click A for a decision tree.
Quality Improvement (QI) consists of systematic and continuous actions that lead to measurable improvement in health care services and the health status of targeted patient groups (HRSA). Systematic: Having or involving a system, method, or plan Action: the fact or process of doing something, typically to achieve an aim Measurable improvement: quantifiable positive change Health care services: medical care or utility/facility Health status of targeted patient groups: the state of body and mind for specific groups of people within a specific setting
A better way to think of it is QI is when you systematically apply what is already known into the local practice, intended to quickly improve patient care/system within a specific setting.
For even more information about QI, here are some supporting documents:
To help you determine if your study is Research or QI, click here for a decision tree.
Non-Human Subjects Research (NHSR) Some projects that are commonly called research are not defined as human subjects research by federal definition and do not require IRB approval. Determining if a project is human subjects research can be challenging. Unfortunately there is no definitive list. The definitions are intentionally broad to capture a wide range of research needs from the biomedical to social and behavioral. Basically, NHSR is research that doesn't involve human subjects or living individuals. It can include research that uses de-identified or coded private information or biological specimens, or research that involves deceased individuals.
In order for a project to not meet the definition of a human subject, all of the following conditions must apply:
- The research is not FDA-regulated.
- The research team will not have access to identifiers or keys to link coded data (even temporarily).
There are many similarities between Research and QI, including:
- Both involve a systematic investigation that is carefully designed to achieve reliable and valid results;
- Both involve analysis of data;
- Both may involve the implementation of a new intervention; and
- Both may result in a presentation or publication.
There are differences between Research and QI, including:
- QI projects are often flexible and incremental in design;
- QI uses data analysis to find out whether or not the workforce is following best practices and professional guidelines;
- QI implements a new practice or process to improve workflow, patient safety, staff expertise, cost effectiveness, etc.;
- QI interventions often have been proven to be successful elsewhere and are widely accepted in the profession/discipline. QI projects evaluate the best strategies to implement these interventions locally;
- QI projects can help us characterize our population in order to better serve their needs or improve their care;
- QI projects aim to directly benefit existing patients by implementing immediate local improvements;
- QI projects do not increase risk to patients beyond the risks that are involved in care they are already receiving;
- QI tools are applicable primarily to the unique characteristics of our local setting; and
- The results of QI projects typically are evaluated by an internal committee or executives who decide whether or not to permanently adopt the new practice.
In addition to the above, further clarification between Research and QI include:
Informed consent
Informed consent is not required for quality improvement projects since they pose only minimal risk. Because quality improvement is an integral aspect of normal health care operations, consent to be included in QI projects is part of the patient’s consent to receive treatment.
Presentations and Publications
The federal agency overseeing research has stated that the act of presenting or publishing a quality improvement project does not change its classification to be research. If you are submitting to a journal that requests an IRB approval, please reach out to clinical.research@vcuhealth.
Generalization
Generalizability is the ability to apply the results of a study to a broader population, such as patients seeking care. It's also known as external validity. Generalizability is an important goal because it allows researchers to draw conclusions that can be useful for the larger community. If your project is designed to develop or contribute to generalizable knowledge (meaning applicable to other institutions, settings, or larger community) then your project may be research. If it not designed to do so but to meet the needs of a specific organization aligned with that organization’s specific policies, procedures, and needs and intended solely for improvement of that organization, then your project may be QI.
Now that you have determined if your project is Research, QI or NHSR, what are the next steps?